Neurologic Complications of Systemic Cancer
- HERBERT B. NEWTON, M.D.
- Ohio State University Hospitals and Arthur James Cancer Hospital and Research Institute
- Columbus, Ohio
Neurologic complications occur frequently in patients with cancer. After routine chemotherapy, these complications are the most common reason for hospitalization of these patients. Brain metastases are the most prevalent complication, affecting 20 to 40 percent of cancer patients and typically presenting as headache, altered mental status or focal weakness. Other common metastatic complications are epidural spinal cord compression and leptomeningeal metastases. Cord compression can be a medical emergency, and the rapid institution of high-dose corticosteroid therapy, radiation therapy or surgical decompression is often necessary to preserve neurologic function. Leptomeningeal metastases should be suspected when a patient presents with neurologic dysfunction in more than one site. Metabolic encephalopathy is the common nonmetastatic cause of altered mental status in cancer patients. Cerebrovascular complications such as stroke or hemorrhage can occur in a variety of tumor-related conditions, including direct invasion, coagulation disorders, chemotherapy side effects and nonbacterial thrombotic endocarditis. Radiation therapy is the most commonly employed palliative measure for metastases. Chemotherapy or surgical removal of tumors is used in selected patients.
TABLE 1
Primary Sites of Metastatic Brain Tumors
Primary tumor
Percentage of patients
Lung 50 to 60 Breast 15 to 20 Melanoma 5 to 10 Gastrointestinal 4 to 6 Genitourinary 3 to 5 Other 3 to 5 Unknown 4 to 8
Information from references 5, 6 and 8.Family physicians often perform the initial evaluation of patients with neurologic complications of systemic cancer.1-4 These complications are diverse and can affect any level of the central and peripheral nervous system. The most frequently implicated tumors are those from the lung, breast, colon, rectum, prostate gland, head and neck, as well as tumors related to leukemia and lymphoma.1,4 Direct involvement of the nervous system includes brain metastases, epidural spinal cord compression, leptomeningeal metastases and various neuropathies (e.g., cranial or peripheral). Indirect effects of systemic cancer include vascular disorders, infections, metabolic abnormalities and paraneoplastic syndromes.
An estimated 15 to 20 percent of cancer patients have symptomatic neurologic complications during the course of their illness.4 The most common complaints are back pain, mental status changes, headache, limb pain and leg weakness. Other than routine chemotherapy, neurologic problems are the most common reason for the hospitalization of patients with systemic cancer.4 Because of improved cancer treatments and longer survival in more patients, neurologic problems will continue to increase in frequency.1,2
Neurologic complications in cancer patients may be even more common than estimates indicate. Investigators at the Johns Hopkins Oncology Center reported that 46 percent of patients admitted to their solid tumor service over a three-month period required either evaluation or treatment of a neurologic problem.1 Another study found that approximately 30 percent of patients with small-cell lung cancer had serious neurologic complications during the course of their disease.2
Patients with neurologic complications of systemic cancer can experience severe weakness, dementia, seizure activity, loss of ambulation, pain and incontinence. Any of these problems can be devastating to functional ability and quality of life. Early recognition and accurate diagnosis, followed by appropriate therapy, often result in pain relief, improved neurologic function, enhanced quality of life and, possibly, prolonged survival.1
Brain Metastases
Brain metastases are the most common neurologic complication of systemic cancer in adult patients.5,6 In the United States, these metastases occur in 20 to 40 percent of cancer patients who are over 20 years of age.6 Metastatic brain tumors are almost 10 times more common than primary brain tumors.7
Other than routine chemotherapy, neurologic problems are the most common reason for the hospitalization of cancer patients. Although metastases to the brain can be generated by primary tumors in a variety of sites (Table 1),5,6,8 more than 60 percent derive from tumors of the lung and breast. Although malignant melanoma represents only 1 percent of all systemic malignancies, it has the highest propensity for spread to the brain.5
Most systemic tumor cells reach the brain by hematogenous spread through the arterial circulation.5,6,8 Occasionally, these cells can reach the brain by way of Batson's plexus (a venous system that drains the base of the skull, spinal column and pelvis) or by direct extension from adjacent structures such as the sinuses or skull.
Brain metastases disrupt the function of adjacent neural tissue by a number of mechanisms, including direct displacement of brain structures by the enlarging tumor, perilesional edema, irritation of overlying gray matter and compression of arterial and venous vasculature.
TABLE 2
Neurologic Signs and Symptoms of Metastatic Brain Tumors
Signs and symptoms
Percentage of patients
Hemiparesis 55 to 60 Impaired cognition 55 to 60 Headache 25 to 40 Focal weakness 20 to 30 Altered mental status 20 to 25 Sensory loss 20 Papilledema 20 Seizure activity 15 to 20 Gait abnormality 15 to 20 Aphasia 15 to 20 Gait disturbance 10 to 20 Speech difficulty 5 to 10 Visual disturbance 5 to 8 Hemianopsia 5 to 7 Limb ataxia 5 to 7 Sensory disturbance 5 Nausea/vomiting 5 Somnolence 5 None 5 to 10
Information from references 5, 6, 8 and 9.Patients with brain metastases may have a variety of neurologic signs and symptoms (Table 2).5,6,8,9 The most common symptoms are headache, altered mental status and focal weakness. The headaches are usually generalized, often occur during sleep and become progressively more severe. Mental status changes may initially be subtle, with patients exhibiting lethargy, loss of interest in activities, irritability or memory loss. The type and degree of weakness depends on the location of the tumor, but a hemiparetic pattern is most common. Seizures are another commonly encountered symptom of brain metastases.
The neurologic examination reveals hemiparesis and impaired cognition in more than 50 percent of patients with brain metastases.5,6,8 Sensory loss or gait abnormalities typically involve one side of the body because of tumor-induced malfunction of the corresponding cerebral hemisphere.
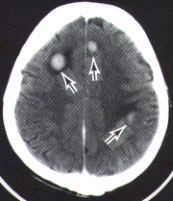
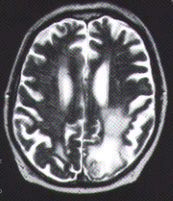
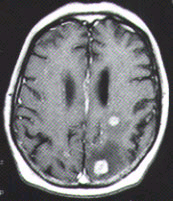
The diagnosis of metastatic brain tumors can be confirmed by enhanced computed tomographic (CT) scanning or magnetic resonance imaging (MRI)5,6 (Figure 1). On both CT and MRI scans, metastatic tumors are typically rounded, well-circumscribed, noninfiltrative masses surrounded by a large amount of edema. A contrast medium almost always enhances the lesion. Although CT scanning remains an excellent screening tool, MRI is more sensitive for detecting multifocal or small tumors, as well as lesions in the cerebellum and brain stem. In some patients, surgical biopsy may be necessary to establish a definitive diagnosis.
In addition to metastatic tumor, the differential diagnosis of a brain lesion (especially a solitary one) includes primary brain tumor, abscess, infarct and hemorrhage.5-7,10,11 A metabolic encephalopathy should be considered in any cancer patient with impaired cognition and no evidence of metastatic lesions on brain imaging. In some studies, metabolic encephalopathy was the most common nonmetastatic complication.10 Encephalopathy can have a variety of causes, including hypercalcemia, electrolyte shifts and chemotherapy.
The initial treatment of patients with brain metastases is directed at controlling cerebral edema and seizure activity.5,6,8 Corticosteroid therapy (e.g., dexamethasone, 2 to 4 mg four times daily) should be started in most patients with metastatic tumors.5,6 Anticonvulsant drugs such as phenytoin (Dilantin) or carbamazepine (Tegretol) are required in patients with generalized or focal seizures.
Additional therapy often includes irradiation and, in selected patients, surgical extirpation and chemotherapy. Surgical removal of an MRI-documented solitary metastasis should be considered.5,6,8,9 Recent trials have demonstrated extended survival after resection.5,6,8,9 Unfortunately, only 25 to 40 percent of patients have single lesions, and many of these patients have other factors that preclude surgery (e.g., inaccessible tumor, extensive systemic disease or other medical problems).5,6
Irradiation is the primary form of therapy for most patients with brain metastases.5,6,8 Generally, this treatment is also used in the small subgroup of patients who have undergone surgical resection. The most common regimen is 3,000 cGy of radiation delivered in 10 treatments over a period of two weeks.
Another promising form of radiation therapy is stereotactic radiosurgery. With this technique, more precise focusing of the radiation beam limits damage to adjacent brain tissue.5,6 Stereotactic radiosurgery is especially useful in patients with small tumors and a favorable prognosis.
FIGURE 1. Imaging studies of two patients with brain metastases. (Left) Contrast-enhanced CT scan shows three separate tumor nodules (arrows)in a 42-year-old woman with breast cancer who developed headaches and confusion. (Center) Unenhanced T2-weighted MRI scan in a 65-year-old man with lung cancer who developed headaches, confusion and memory loss. Extensive high-signal abnormalities are present in the left posterior parietal and occipital lobes. A circular mixed-signal lesion is noted at the occipital pole. (Right) Contrast-enhanced T1-weighted MRI scan of the same man, showing two enhancing nodules of tumor surrounded by low-signal edema. In both patients, note the circular, circumscribed nature of the tumors and the significant edema.Epidural Spinal Cord Compression
Back pain is a common problem with an annual incidence of 5 percent and a lifetime prevalence of 60 to 90 percent in the general U.S. population.12 In most people, back pain is benign and self-limited. However, in patients with systemic cancer, back pain can be the first sign of a serious underlying neurologic process. The structural components of the spinal column are the most common sites for bony metastases, which often occur in patients with cancer of the prostate gland, breast, kidneys or lungs, as well as in patients with melanoma or myeloma.13,14
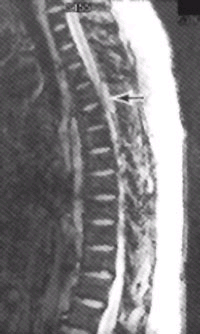
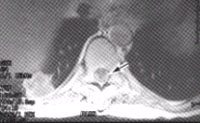
FIGURE 2. T2-weighted MRI scan of the spine in a 53-year-old woman with non-Hodgkin's lymphoma who developed back pain and mild leg weakness. A high-signal mass (arrow) is compressing and displacing the thoracic spinal cord.Epidural spinal cord compression (ESCC), the most dreaded sequela of spinal column metastasis, is relatively common, occurring in 5 to 14 percent of patients with systemic cancer.14,15 Although ESCC usually develops in patients with an existing diagnosis of cancer and widespread disease, it may be the first manifestation of cancer in up to one fourth of patients. Once back pain develops, neurologic deterioration can be rapid. Therefore, patients with ESCC quickly develop paraplegia, loss of lower extremity sensation and/or incontinence.
The differential diagnosis of back pain is broad,12,13 but new-onset back pain in a cancer patient most commonly has a tumor-related cause.1,4 A thorough history should be obtained, with special attention given to associated neurologic signs or symptoms. In most patients with ESCC, pain is the initial symptom and develops anywhere along the spine, most often in the thoracic region.15,16 The pain is mild at first but is always progressive.
After the onset of pain, other common symptoms develop. Extremity weakness and autonomic dysfunction occur in 75 and 55 percent of patients, respectively.13,14,16 Weakness is usually symmetric and involves the legs, although it occasionally affects the arms. Autonomic dysfunction manifests in most patients as painless urinary retention, with urinary and bowel incontinence less frequently noted. Sensory complaints develop concomitantly with weakness. These complaints usually manifest as numbness and paresthesias that start in the feet and extend proximally over time.
Certain features help to differentiate ESCC from other conditions. The presence of fever (especially with a history of recent sepsis) suggests epidural abscess, diskitis, osteomyelitis or some other infectious process.
On physical examination, patients with ESCC often demonstrate localized pain to percussion over the involved vertebral bodies.13,17 Most commonly, the tender areas are in the thoracic region; in contrast, the pain of degenerative spinal disease is more often cervical or lumbosacral.
Up to 40 percent of cancer patients develop brain metastases during the course of their illness. The neurologic examination most often demonstrates leg weakness.13,17 During the early phase of ESCC, the findings may be mild, and patients may have weakness of the iliopsoas and hamstring muscle groups, along with slightly exaggerated patellar and Achilles reflexes. As ESCC progresses, patients exhibit an upper motor neuron pattern with weakness accompanied by spasticity, Babinski's sign and exaggerated reflexes. ESCC of the lumbar vertebrae affects the cauda equina instead of the spinal cord (the spinal cord ends at L1). This produces a lower motor neuron pattern characterized by hypotonia, areflexia, muscle atrophy and fasciculations. Early sensory findings usually consist of a mild decrease in distal vibratory and proprioceptive sensation, whereas more advanced disease is characterized by loss of light touch and pinprick below the level of ESCC.
ESCC can arise from metastases to the vertebral column (85 percent of cases), paravertebral spaces (10 to 12 percent of cases) or epidural space (1 to 3 percent of cases).13 Enlargement of vertebral metastases initially causes local pain resulting from stretching of the periosteum. Further growth compresses adjacent neural and vascular structures, evoking additional neurologic signs and radicular pain. Compromise of the spinal arterial blood supply and vertebral venous plexus can induce vasogenic edema, hemorrhage, demyelination, ischemia and infarction within the spinal cord parenchyma.13
TABLE 3
Presenting Signs and Symptoms of Leptomeningeal Metastases
Signs and symptoms
Percentage of patients
Reflex asymmetry 60 to 70 Weakness 45 to 70 Altered mental status 40 to 60 Cranial nerve palsy 40 to 50 Headache 30 to 45 Spinal or radicular pain 20 to 40 Sensory deficits or alterations 20 to 35 Gait abnormalities 15 to 30 Nausea and vomiting 10 to 30 Cerebellar signs or ataxia 15 to 25 Bowel or bladder dysfunction 15 to 20 Spells or seizures 16 Miscellaneous* 5 to 15
*--This category includes vision problems, dysphagia, meningismus and others.
Information from references 22, 23 and 24.
The initial management of ESCC consists of pain control and diagnostic evaluation. The pain is often severe, and patients may require parenteral narcotic analgesics for adequate control. Plain radiographs of the spine identify an abnormality in 85 to 90 percent of patients with ESCC resulting from solid tumors.13 The most common lesions are vertebral body erosion and collapse, subluxation and pedicle erosion. Recently, MRI has replaced myelography as the most sensitive and specific imaging technique for the evaluation of epidural tumor13,17 (Figure 2).
Patients suspected of having ESCC must be evaluated rapidly because the most important prognostic factor for the preservation of neurologic function is the degree of function at the initiation of therapy.13 Approximately 80 percent of patients who are ambulatory at the start of treatment remain so after therapy, but only 5 to 10 percent of paraplegic patients are ambulatory after treatment.13,16,18 A corticosteroid should be administered intravenously to patients with proven ESCC or in whom ESCC is strongly suspected on clinical grounds. High-dose dexamethasone rapidly reduces spinal cord edema and may improve neurologic function and back pain. A regimen consisting of a loading dose of 20 to 100 mg, followed by 4 to 24 mg four times daily, is often used.14,18 Within 24 hours of the initiation of steroid treatment, radiation therapy is usually started or surgical decompression is performed.
The role of decompressive surgery in the treatment of ESCC is poorly defined. Surgical resection may be considered if neurologic deterioration occurs despite radiation therapy, the involved tumor is known to be radioresistant (e.g., renal) or acute deterioration of neurologic function occurs.13,17
Radiation therapy, the solitary modality of treatment for the majority of patients with ESCC, is also necessary after surgical decompression. Most commonly, a total of 3,000 cGy is administered in 10 daily fractions.18 Radiation therapy or surgical decompression may be considered even when severe deficits have been present for several days or more, because these treatments may improve neurologic function in some patients.
Cerebrovascular Complications
Autopsy studies reveal cerebrovascular lesions in approximately 15 percent of all cancer patients.10,11,19 Although large-vessel atherosclerotic disease is common in cancer patients, tumors are responsible for most symptomatic infarctions.19 Cerebrovascular disease in cancer patients can be caused by a tumor directly compressing or invading blood vessels, tumor-induced coagulation disorders (hemorrhagic and thrombotic) or treatment-related injury to blood vessels.11,19
Approximately 25 percent of strokes in cancer patients are caused by nonbacterial thrombotic endocarditis.10,11,19 This type of endocarditis is characterized by sterile fibrin vegetations on one or more cardiac valves. Embolization of the vegetations often manifests with focal symptoms suggestive of a classic transient ischemic attack or stroke. However, patients may also have a more diffuse presentation consistent with encephalopathy or confusion. In some patients, nonbacterial thrombotic endocarditis can be diagnosed with the use of transesophageal echocardiography.
Leptomeningeal Metastases
Epidural spinal cord compression, the most dreaded sequela of spinal column metastasis, is relatively common. Leptomeningeal metastases, also known as meningeal carcinomatosis or neoplastic meningitis, develop when cancer cells spread into the cerebrospinal fluid (CSF) of the subarachnoid space, which bathes the brain, spinal cord and spinal nerve roots.20-23 Like parenchymal brain metastases, leptomeningeal metastases are most commonly caused by breast cancer, lung cancer and malignant melanoma.
The exact incidence of leptomeningeal metastases is unknown, but autopsy studies suggest an overall incidence of 5 to 8 percent in patients with systemic cancer.20,23 In most patients, these metastases are a late manifestation of progressive, widespread disease. Leptomeningeal metastases should always be suspected in a cancer patient with neurologic signs and symptoms indicating dysfunction in more than one anatomic site within the nervous system (e.g., seizures and diminished leg reflexes).
The spine is affected in 75 to 80 percent of patients with leptomeningeal metastases.20,23 Spinal signs and symptoms include neck or back pain, asymmetric reflexes or extremity weakness. Pain is often prominent and can present as neck stiffness, localized spinal tenderness or radicular discomfort that radiates from the spine into an arm or leg (Table 3).22-24 Weakness is also common and usually affects both legs in a lower motor neuron pattern (i.e., flaccid tone, loss of reflexes, atrophy, fasciculations and negative Babinski's sign). More than one half of patients with leptomeningeal metastases have cranial nerve palsies.22-24 Common symptoms include diplopia, visual loss, dysphagia, hearing loss and facial numbness.
FIGURE 3. Contrast-enhanced T1-weighted axial MRI scan of the spine in a 33-year-old woman with breast cancer who developed low back pain, leg weakness and numbness. The superficial nodular enhancement around the spinal cord (arrow) is consistent with leptomeningeal metastases.The brain is affected in approximately one half of patients with leptomeningeal metastases.22-24 The most common symptoms are headache, cognitive changes, gait abnormalities, seizures and nausea and vomiting. Mental status and cognitive changes often occur as the leptomeningeal tumor grows over the cerebral hemispheres, causing bilateral cortical dysfunction.
The diverse mechanisms by which leptomeningeal metastases produce neurologic dysfunction include the following: elevation of intracranial pressure; direct invasion into brain, spinal cord or nerves; obstruction of penetrating meningeal arterial vessels, and metabolic alterations.
The most important diagnostic tests are lumbar puncture and contrast-enhanced MRI studies of the brain and/or spine.22,23 In selected patients, it may be prudent to rule out a mass lesion with an MRI scan before lumbar puncture is performed. If the MRI study is unequivocally positive for leptomeningeal metastases, lumbar puncture may be unnecessary. CSF cytologic studies are positive in 45 to 50 percent of patients after one lumbar puncture and in more than 90 percent of patients after three lumbar punctures.20
Contrast-enhanced MRI of the brain and/or spine demonstrates the presence of meningeal tumor in 30 to 50 percent of patients.23 The disrupted bloodCSF barrier leads to enhancement of neoplastic meningeal vessels, which appear as either a thin rind or multifocal nodules over the brain or spinal cord (Figure 3). Enhancement in a pattern consistent with the patient's clinical findings is often considered sufficient evidence of leptomeningeal metastases to justify initiation of treatment, even if CSF cytologic studies are negative. Communicating hydrocephalus can also be a sign of leptomeningeal metastases, because meningeal tumor often impairs CSF pathways.
Leptomeningeal metastases are treated with a combination of radiation therapy and intrathecal chemotherapy.20-23,25 Radiation therapy can be directed at the entire neuraxis, symptomatic sites or areas of enhancing bulky disease. Focal radiation therapy to the brain or spine is generally recommended for patients with symptomatic disease. Intrathecal chemotherapy is given by lumbar puncture or with a ventricular access device such as an Ommaya reservoir. Use of an Ommaya reservoir may help to provide more uniform drug concentrations throughout the neuraxis.
Neuromuscular Complications
Cancer can affect nerves and muscles as a result of direct infiltration or compression by a tumor, as a side effect of cancer treatment or as a paraneoplastic effect of cancer24,26 (Table 4). As discussed previously, cranial neuropathies are most commonly caused by leptomeningeal metastases within the subarachnoid space. However, tumors can also damage cranial nerves after they have exited the subarachnoid space. For example, nasopharyngeal carcinoma presents with cranial nerve palsies in 15 to 30 percent of patients.27 Breast, lung and prostate gland cancers often metastasize to bone, and lesions at the base of the skull can cause cranial nerve dysfunction.26 The "numb chin" syndrome, or mental neuropathy, is caused by dysfunction of the inferior alveolar nerve (a branch of cranial nerve V) as it courses through the mandible.28 Patients with this syndrome complain of numbness (without pain) over the chin and lower lip. Mandibular and leptomeningeal metastases are usually responsible.
TABLE 4
Complications Affecting Nerves and Muscles in Cancer Patients
- • Cranial neuropathies
- • Brachial and lumbosacral plexopathy
- --Neoplastic plexopathy
- --Radiation-induced plexopathy
- • Chemotherapy-related neuropathies
- • Paraneoplastic neuropathies
- • Paraneoplastic disorders
- --Inflammatory myopathies
- --Lambert-Eaton syndrome
Enhanced MRI studies can be helpful in determining the etiology of a cranial neuropathy syndrome. Treatment consists of radiation therapy focused into the symptomatic region.
Although brachial and lumbosacral plexopathies are most often caused by tumor infiltration or compression, similar clinical syndromes can develop as a consequence of radiation therapy.24 Neoplastic brachial plexopathy is usually caused by an apical lung mass (i.e., Pancoast's tumor) or breast cancer that has metastasized to axillary lymph nodes. As the tumor or lymph nodes enlarge, the plexus is invaded or compressed from below. The initial symptom is usually a dull, aching pain involving the shoulder and arm. The pain becomes progressively more severe and later is often accompanied by numbness, paresthesias and weakness of the arm or hand. The clinical presentation of a fibrotic plexopathy related to radiation for breast carcinoma is quite similar.24
The diagnosis can be made with contrast-enhanced CT scans or MRI studies of the brachial plexus region. Electromyography can be helpful in localizing the disease process within the plexus. Radiation therapy is used in most patients with neoplastic brachial plexopathy.
Lumbosacral plexopathy is most commonly caused by direct extension of local tumor masses from colorectal, cervical and prostate cancers. Incontinence and impotence may develop. The diagnosis and treatment of lumbosacral plexopathy are comparable to those for brachial plexopathy.24
Up to 25 percent of strokes in cancer patients are caused by nonbacterial thrombotic endocarditis. Chemotherapy-induced peripheral neuropathies are a common side effect and cause of morbidity in cancer patients.24 The agents most often associated with neuropathy are vincristine (Oncovin) and cisplatin (Platinol). In most patients, the initial complaint is tingling and paresthesias of the distal extremities. Reflexes disappear, and vibratory and proprioceptive capacity is reduced. Recovery is variable after the agent is discontinued.
This work was supported in part by National Cancer Institute Grant CA 16058.
The author thanks David Pfalzer, M.D., and Harrison Weed, M.D., for critical review of the manuscript, and David Carpenter, for editorial expertise.
The Author
HERBERT B. NEWTON, M.D.,
is associate professor of neurology and director of the neuro-oncology division at the Ohio State University Hospitals and Arthur James Cancer Hospital and Research Institute, Columbus, Ohio. After earning his medical degree from the State University of New York at Buffalo School of Medicine, Dr. Newton completed a residency in neurology at the University of Michigan Medical Center, Ann Arbor, and a fellowship in neuro-oncology at Memorial Sloan-Kettering Cancer Center, New York City.Address correspondence to Herbert B. Newton, M.D., Division of Neuro-Oncology, Department of Neurology, Ohio State University Hospitals, 465 Means Hall, 1654 Upham Dr., Columbus, OH 43210. Reprints are not available from the author.
REFERENCES
- Gilbert MR, Grossman SA. Incidence and nature of neurologic problems in patients with solid tumors. Am J Med 1986;81:951-4.
- Sculier JP, Feld R, Evans WK, DeBoer G, Shepherd FA, Payne DG, et al. Neurologic disorders in patients with small cell lung cancer. Cancer 1987;60:2275-83.
- Hovestadt A, van Woerkom TC, Vecht CJ. Frequency of neurological disease in a cancer hospital [Letter]. Eur J Cancer 1990;26:765-6.
- Clouston PD, DeAngelis LM, Posner JB. The spectrum of neurological disease in patients with systemic cancer. Ann Neurol 1992;31:268-73.
- O'Neill BP, Buckner JC, Coffey RJ, Dinapoli RP, Shaw EG. Brain metastatic lesions. Mayo Clin Proc 1994;69:1062-8.
- Patchell RA. The treatment of brain metastases. Cancer Invest 1996;14:169-77.
- Newton HB. Primary brain tumors: review of etiology, diagnosis, and treatment. Am Fam Physician 1994;49:787-97.
- Nussbaum ES, Djalilian HR, Cho KH, Hall WA. Brain metastases. Histology, multiplicity, surgery, and survival. Cancer 1996;78:1781-8.
- Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med 1990;322:494-500.
- Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis. Clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746-56.
- Rogers LR. Cerebrovascular complications in cancer patients. Oncology 1994;8:23-30.
- Frymoyer JW. Back pain and sciatica. N Engl J Med 1988;318:291-300.
- Byrne TN. Spinal cord compression from epidural metastases. N Engl J Med 1992;327:614-9.
- Sioutos PJ, Arbit E, Meshulam CF, Galicich JH. Spinal metastases from solid tumors. Analysis of factors affecting survival. Cancer 1995;76:1453-9.
- Klein SL, Sanford RA, Muhlbauer MS. Pediatric spinal epidural metastases. J Neurosurg 1991;74:70-5.
- Bach F, Agerlin N, Sorensen JB, Rasmussen TB, Dombernowsky P, Sorensen PS, et al. Metastatic spinal cord compression secondary to lung cancer. J Clin Oncol 1992;10:1781-7.
- Forman AD. Neurologic emergencies in cancer patients. Cancer Bull 1992;44:197-206.
- Maranzano E, Latini P, Checcaglini F, Ricci S, Panizza BM, Aristei C, et al. Radiation therapy in metastatic spinal cord compression. A prospective analysis of 105 consecutive patients. Cancer 1991;67:1311-7.
- Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine 1985;64:16-35.
- Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer 1982;49:759-72.
- Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 1990;9:225-9.
- Boogerd W, Hart AAM, van der Sande JJ, Engelsman E. Meningeal carcinomatosis in breast cancer. Prognostic factors and influence of treatment. Cancer 1991;67:1685-95.
- Balm M, Hammack J. Leptomeningeal carcinomatosis. Presenting features and prognostic factors. Arch Neurol 1996;53:626-32.
- Chad DA, Recht LD. Neuromuscular complications of systemic cancer. Neurol Clin 1991;9:901-18.
- Grant R, Naylor B, Greenberg HS, Junck L. Clinical outcome in aggressively treated meningeal carcinomatosis. Arch Neurol 1994;51:457-61.
- Greenberg HS, Deck MD, Vikram B, Chu FC, Posner JB. Metastasis to the base of the skull: clinical findings in 43 patients. Neurology 1981;31:530-7.
- Stillwagon GB, Lee DJ, Moses H, Kashima H, Harris A, Johns M. Response of cranial nerve abnormalities in nasopharyngeal carcinoma to radiation therapy. Cancer 1986;57:2272-4.
- Lossos A, Siegal T. Numb chin syndrome in cancer patients: etiology, response to treatment, and prognostic significance. Neurology 1992;42:1181-4.
Copyright © 1999 by the American Academy of Family Physicians.
This content is owned by the AAFP. A person viewing it online may make one printout of the material and may use that printout only for his or her personal, non-commercial reference. This material may not otherwise be downloaded, copied, printed, stored, transmitted or reproduced in any medium, whether now known or later invented, except as authorized in writing by the AAFP.